Edwards Lifesciences IntraClude device
Minimal Incision Valve Surgery
Minimal incision valve surgery (MIVS) provides excellent outcomes and significant benefits for patients and surgeons alike. Through peripheral cannulation, Edwards ThruPort systems’ allows for fewer products within the incision site. This offers surgeons excellent visualization and a virtually bloodless, unobstructed operative field , enabling mitral valve repair or replacement through the smallest possible incision.
The IntraClude intra-aortic occlusion device is designed for:
- Less traumatic intra-aortic occlusion
- Antegrade cardioplegia delivery
- Aortic root venting
- Aortic root pressure monitoring
Thruport IntraClude Intro-Aortic Occlusion Device
The IntraClude device can be used in cardiopulmonary bypass procedures such as minimal incision mitral valve repair or replacement procedures, re-operations, tricuspid valve procedures, intracardiac myxoma resection, patent foramen ovale repairs, atrial septal defect repairs, and ablative maze procedures for atrial fibrillation.
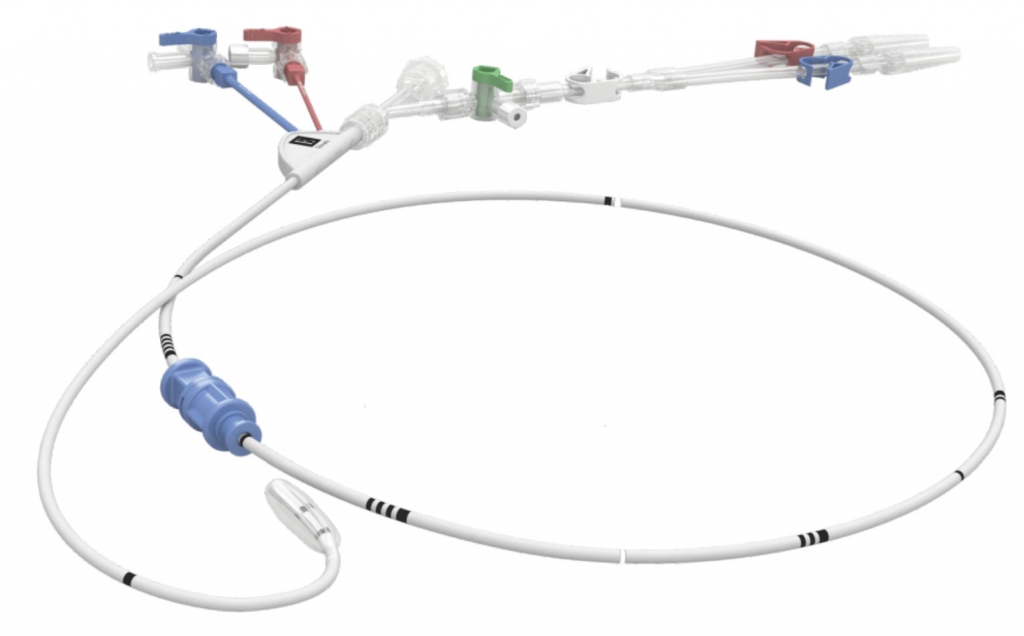
The IntraClude device is designed for use with cardiopulmonary bypass and features:
10.5 Fr, triple lumen catheter
- Central lumen provides for antegrade cardioplegia delivery, aortic root venting and guidewire passage
- Blue lumen enables balloon inflation/deflation
- Red lumen enables reliable aortic root pressure monitoring
100 cm in length with an elastomeric balloon at the tip
- Balloon expands to occlude a range of aorta sizes (2.0 cm to 4.0 cm)
Packaging also includes:
- 200 cm 0.038″ J-tip guidewire 35 mL syringe
- Y-connector
- Red and blue pressure lines
