InQu® Bone Graft Extender & Substitute
FASTER FUSION AND BONE FORMATION
A safe and cost-effective bone graft substitute and extender, InQu creates a natural microenvironment supporting new bone formation in spinal fusions and orthopedic procedures.
Surgeons use bone grafts to generate new bone, repair bone defects resulting from trauma and osteotomy, and create spinal fusions. However, using autografts from a patient’s body risks chronic pain and potential infection at the donor site. As a result, surgeons regularly employ a variety of synthetic bone graft substitutes, commonly ceramic and calcium salt-based. Unfortunately, ceramic-based bone grafts lack ideal handling properties and predictable absorption rates.


Isto’s Solution
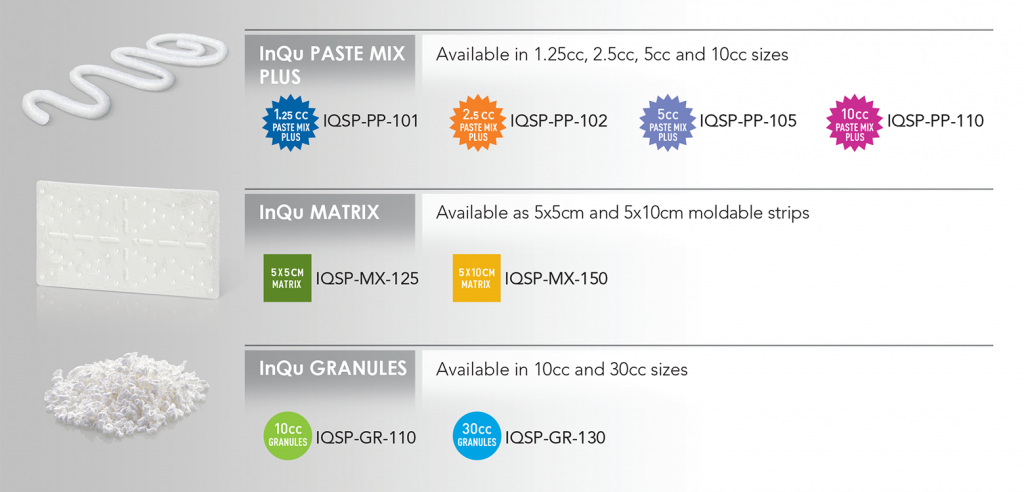
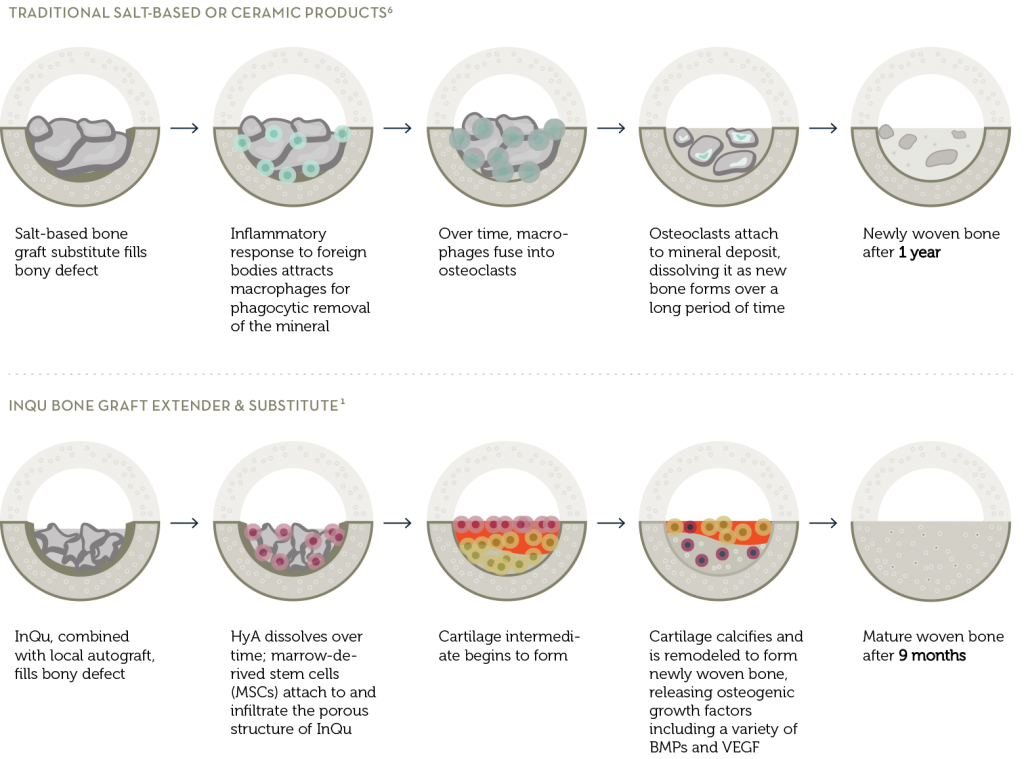
InQu was strategically designed as an osteoconductive biosynthetic that provides surgeons with a safe, direct, and rapid path to new bone formation. Unlike other bone graft synthetics, which require a period of mineral breakdown before new bone can begin to form, InQu partners with the body’s natural processes to create new bone in a shorter period of time with no inflammatory response1. InQu is intended for use as a bone graft substitute in the skeletal system (extremities and pelvis) and as a bone graft extender in the spine when combined with bone autograft.
InQu does not use human or animal cells or tissue, but rather two biomaterials with a long history of safe and effective clinical use2,3,4, InQu interweaves hyaluronic acid (HyA) into the structural support network of resorbable poly(lactide-co-glycolide) (PLGA). This creates a unique microenvironment with an excellent biocompatibility profile that supports new bone formation and may achieve faster fusion than traditional ceramic bone grafts1.
The unique biosynthetic makeup of InQu has hydrophilic properties, allowing the wicking of blood and other bodily fluids encountered during surgery. Additionally, InQu’s radiolucency allows surgeons to easily distinguish new bone growth on x-ray. InQu’s properties also include excellent compressive resistance similar to that of native trabecular bone5. The rate of InQu resorption and its replacement by newly formed bone is consistent with the rate of bone remodeling at the site of implantation, thus providing the surgeon with predictable results.
Due to its unique structure, InQu provides surgeons with superior cohesiveness, molding properties, and handling characteristics. InQu is available in three distinct, sterile product configurations (Paste Mix Plus, Matrix, and Granules) to deliver flexibility and ease of use. With its maximum versatility, InQu is appropriate for a variety of spinal and orthopedic procedures.
SUCCESS OF FUSION
In a retrospective analysis of 149 spinal fusion procedures, InQu in combination with an autograft demonstrated an overall fusion rate of 93.6% at 12 months7. In a comparative clinical study of InQu vs. tricalcium phosphate in posterolateral spinal fusion, InQu in combination with an autograft achieved a 92.9% successful fusion rate vs. 67.9% for the tricalcium phosphate and autograft group at one year8.